New PNAS paper reveals how a human spleen works
text Heading link

It is common knowledge that the heart pumps blood and the stomach digests food, but very few people can explain what the human spleen is for. Scientists can tell you that a major function of the spleen is to remove aged and abnormal red blood cells from circulation and recycle them, acting like a blood filter. However, the exact way the spleen can filter these cells remains a mystery.
To solve this puzzle, a new paper published in the Proceedings of the National Academy of Sciences (PNAS) by Richard and Loan Hill Department of Biomedical Engineering Assistant Professor Zhangli Peng and his collaborators Annie Viallat and Emmanuèle Helfer from Aix Marseille University in France combined microfluidic experiments and multiscale modeling to reveal the physical mechanisms of this filtration process.
Peng and his team discovered that 8-micron healthy red blood cells can pass through 0.28-micron rigid slits in a microfluidic device they designed to mimic the spleen without any problem or damage at 37 degrees Celsius, which is the average human body temperature. Interestingly, they found that at room temperature a significant amount of red blood cells became stuck in the slits.
Anyone who has tried to move a couch through a small doorway knows how difficult it can be to try and move a large object through a smaller opening, so how exactly is the red blood cell able to pass through such small slits? Peng explained that at body temperature the cell’s cytoskeleton unfolds, allowing the cell to deform into a shape close to two spheres connected by a narrow tether, which in principle can pass through any gap no matter how small it is.
block Heading link
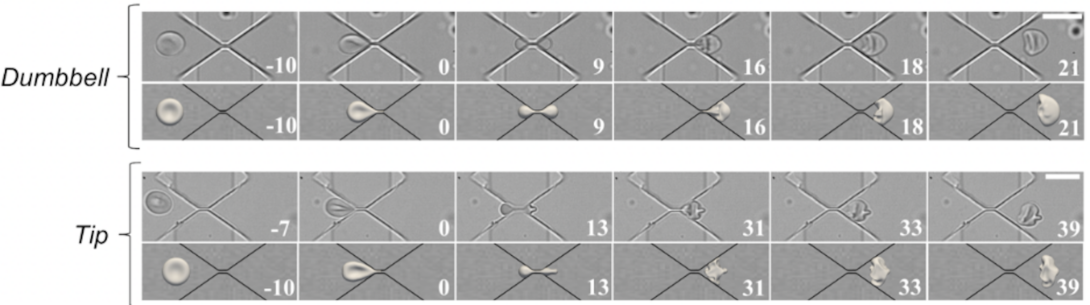
The researchers also investigated how fast the cell passes through a gap and its dependence on pressure, gap size, temperature, and cell geometry, all to better understand how the process actually works in the human body. The computationally predicted passing speed matches well with the experimental measurements, and also provides quantitative insights on the dynamics of the process.
“This is a major milestone because many people have simulated this cell path, but no one has previously validated the path and components of the cells,” Peng said. “This is the first systematic comparison between a real experiment and simulation.”
Besides splenic filtration of red blood cells, the integrated approach of microfluidic experiments and multiscale modeling used by Peng and his team can be applied to study many other important physiological processes. For example, Peng and his colleagues Richard and Loan Hill Professor Ian Papautsky and Professors Asrar Malik and Yulia Komarova’s groups from the Department of Pharmacology studied neutrophils passing the small gaps between the endothelial cells in the lung, a process called neutrophil diapedesis. By using an integrated in-vivo, in-vitro, and in-silico approach, they found that the activated mechanosensitive channel PIEZO1 on the neutrophil membranes during diapedesis can greatly enhance its killing capability of bacteria.
In another related project, supported by funding from the NSF and French Agence Nationale de la Recherche, Peng and his French collaborators are investigating the enucleation process of red blood cell generation in controlled microfluidic devices mimicking bone marrow, in which cells also need to pass through the gaps between endothelial cells in bone marrow. The microfluidic device they created will provide a similar mechanical environment to the bone marrow to facilitate the process of enucleation to enhance the efficiency of artificially producing blood. Peng, Viallat, and Helfer are working with Northwestern University Professor of Experimental Pathology and Hematopathology Peng Ji on the research.